Intraosseous Infusion Devices Market Accelerates Across USA, Europe, APAC & Saudi Arabia with 7.5% CAGR to 2035
Intraosseous infusion devices market projected to double to USD 10.46B by 2035, driven by rising emergency cases, smart device adoption, and global healthcare.
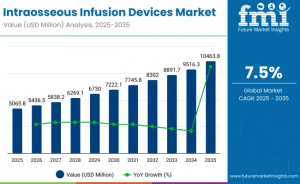
FRANCE, November 10, 2025 /EINPresswire.com/ -- The global intraosseous infusion devices market is positioned for robust growth, with sales expected to increase from USD 5,065.8 million in 2025 to USD 10,463.8 million by 2035, reflecting a steady CAGR of 7.5% during the forecast period. The market recorded revenue of USD 4,725.6 million in 2024, supported by ongoing advancements in emergency medical response systems, rising prevalence of chronic diseases, and expanding healthcare infrastructures across major global regions including the United States, Europe, Asia-Pacific (APAC), and Saudi Arabia.
Intraosseous infusion devices are essential tools used for rapid vascular access when intravenous (IV) access is delayed or unsuccessful, typically in emergency and critical care scenarios. Their design enables direct infusion of fluids and medications into bone marrow, allowing immediate treatment in life-threatening situations such as cardiac arrest, trauma, shock, dehydration, seizures, and complex comorbidities. With emergency medical services (EMS) worldwide adopting faster intervention protocols, these devices are becoming a frontline component of trauma and resuscitation care.
Explore trends before investing — request a sample report today!:- https://www.futuremarketinsights.com/reports/sample/rep-gb-1569
Key Growth Drivers
The escalation in cardiovascular diseases remains a pivotal factor influencing demand. According to the American Heart Association, cardiovascular conditions are responsible for 17.3 million deaths annually and are projected to reach 23.6 million deaths by 2030. Rapid vascular access is critical in cardiac arrest cases, where treatment delays can significantly increase mortality risk. Intraosseous delivery provides an alternative to IV access when time is limited or peripheral veins have collapsed due to shock.
Additionally, sedentary lifestyles and aging demographics are contributing to chronic illnesses such as renal failure, COPD, and metabolic disorders, thereby driving hospitalizations and emergency care requirements. Rising trauma cases from traffic accidents globally and continued expansions in air and ground ambulance services are also expected to support market adoption.
Regional Outlook
• United States: The country leads the market with strong adoption across hospitals and EMS organizations, driven by training integration and updated clinical guidelines. The U.S. market is anticipated to grow at a CAGR of 7.4% between 2025 and 2035.
• Europe: Germany and France demonstrate strong demand due to advanced trauma response systems and high investment in medical innovation. Germany is projected to grow at 8.0% CAGR through 2035.
• Asia-Pacific (APAC): Rapid healthcare modernization in China and India is accelerating device adoption. India is expected to lead the region with a CAGR of 8.7% during the forecast period.
• Saudi Arabia: Increased government spending on emergency medical infrastructure, military healthcare, and advanced trauma centers continues to drive demand.
Click Here to Purchase the Report:- https://www.futuremarketinsights.com/checkout/1569
Product and Technology Trends
The EZ-IO device segment holds the largest product share, accounting for 21.5% in 2025, primarily attributed to its ease of use and rapid access capabilities in under 30 seconds. Sternum-based intraosseous administration remains the most preferred route, capturing 30.9% of the market due to its anatomical accessibility during CPR.
Automated and power-driven intraosseous systems are seeing heightened adoption due to minimal training requirements and increased success rates, particularly in pre-hospital emergency settings and remote environments.
Competitive Landscape
Tier 1 manufacturers including Teleflex, Becton Dickinson (BD), PerSys Medical, and Cook Medical continue to dominate with strong R&D pipelines and global distribution networks. Innovations such as real-time placement feedback, safety-engineered needles, and data-connected infusion systems are shaping next-generation device configurations.
Recent advancements include BD’s introduction of a rechargeable driver-based IO system with integrated needlestick safety enhancements and Teleflex’s continued optimization of its FAST1™ system for sternal access reliability.
Latest Therapeutic Device Reports:-
Balloon Catheters for Bile Stone Removal Market
https://www.futuremarketinsights.com/reports/balloon-catheters-for-bile-stone-removal-market
Smart Wheelchair market
https://www.futuremarketinsights.com/reports/smart-wheelchair-market
Transcatheter Mitral Valve Market
https://www.futuremarketinsights.com/reports/transcatheter-mitral-valve-market
Why Choose FMI Empowering Decisions that Drive Real-World Outcomes:- https://www.futuremarketinsights.com/why-fmi
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analystsworldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.