Ennov Releases Version 11.0, Delivering AI-Powered Productivity Across Its Unified GxP Platform
A smarter, faster, unified way to work across Regulatory, Quality, Clinical, and Pharmacovigilance, now with powerful, built-in AI. By Ennov.
PARIS, FRANCE, December 3, 2025 /EINPresswire.com/ -- Ennov 11.0 introduces built-in AI, a redesigned global homepage, and targeted enhancements across Regulatory, Quality, Clinical, and Pharmacovigilance, helping regulated teams work smarter, faster, and with greater confidence.
Ennov, the only global provider of the AI-powered unified GxP platform for life sciences, announced the availability of Ennov 11.0, its most advanced release to date. The new version brings native AI capabilities directly into daily operations, introduces a powerful, customizable global homepage, and adds a wide range of functional improvements across all domains.
Designed in close partnership with regulatory, quality, clinical, and PV teams worldwide, Ennov 11.0 reflects the vision of long-standing commitment to delivering modern, intuitive, and compliant software solutions for the life sciences industry.
AI at the Core of the Platform
Ennov 11.0 introduces powerful AI capabilities built natively into the unified platform, ensuring full traceability, security, and regulatory compliance. These features require no external tools or integrations and are optimized for GxP environments.
Key capabilities include:
AI chatbot assistant for instant navigation of documents, records, tasks, and actions
Auto-classification of eCTD content and streamlined agency correspondence for Regulatory teams
AI-generated quizzes to accelerate Quality training workflows
Automated case narrative summarization for Pharmacovigilance reviewers
All actions are logged, validated, and fully auditable, ensuring compliance from day one.
“Ennov 11.0 represents a breakthrough in how regulated teams work,” said Olivier Pâris, CEO and Founder of Ennov. “By embedding AI directly into the platform, we are giving our customers a compliant, secure, and practical way to increase productivity across the entire product lifecycle. This is not AI on the side or AI as a plug-in. It is AI embedded into daily work, exactly where teams need it.”
A New Homepage for a Unified Work Experience
The Ennov unified platform gives teams a single place to see their data, documents, and ongoing work. Ennov 11.0 takes that to the next level.
A new, powerful global homepage that brings documents, tasks, signatures, reviews, and messages together in a single, centralized view in a completely customizable way. Here are the biggest benefits:
Role-based widgets for KPIs and operational updates
Actionable task and signature queues in one location
Inline and batch actions to accelerate reviews
Real-time compliance indicators guiding users toward accurate, audit-ready output
“Our focus was to further simplify the experience without reducing capability,” said James Deleuse, Chief Product Officer at Ennov. “Teams now have a single cockpit that brings documents, tasks, reviews, and decisions into one view. This improves alignment and helps users stay ahead of deadlines, audits, and regulatory milestones.”
Enhancements Across Regulatory, Quality, Clinical and PV
Ennov 11.0 includes customer-driven updates across every domain, including:
Regulatory: drag-and-drop dossier reuse, eCTD import capabilities, and an Approved View for instant access to authorized content
Quality: auto-generated training quizzes, linked Quality and Training records, and real-time error-prevention indicators
Clinical: integrated RTSM and EDC, default e-signatures, TEST/PROD separation, and interactive study dashboards
Pharmacovigilance: AI-enabled case summarization and prioritization that requires no process reconfiguration
These enhancements strengthen traceability, improve collaboration, and reduce administrative workload, helping teams focus on higher-value, compliance-critical tasks.
About Ennov
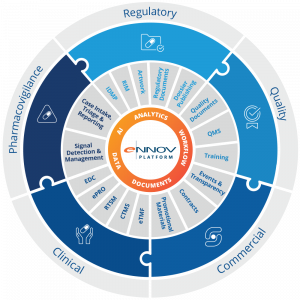
With over 25 years of innovation, Ennov delivers user-friendly, powerful software supporting the entire Life Sciences R&D continuum, including Clinical, Regulatory, Quality, Pharmacovigilance, and Commercial business areas. All Ennov solutions run on a single, unified GxP compliance platform built on shared components such as document management, workflows, advanced data, analytics, and AI, ensuring seamless interoperability and eliminating silos.
Ennov serves more than 450 life sciences organizations and over 500,000 users worldwide. Its team of approximately 450 professionals spans five countries and five continents, providing global reach with local expertise. The company maintains a 98.5 percent on-time delivery rate, a 96 percent maintenance renewal rate, and consistently high user adoption across all solutions. Ennov proudly holds ISO/IEC 27001 and ISO 9001:2015 certifications and has a 100 percent customer audit success rate. The company is recognized by Gartner® across key domains including EDM, RIM, Pharmacovigilance, and eTMF.
Alp Tetikel
Ennov
email us here
Visit us on social media:
LinkedIn
Other
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.